Effective Treatment of Chronic Fatigue Syndrome and Fibromyalgia - A Randomized, Double-Blind, Placebo-Controlled, Intent to Treat Study
[Download a print-friendly pdf version of this report.]
Jacob E. Teitelbaum, MD*1; Barbara Bird, M.T.,C.L.S.*; Robert M. Greenfield, MD1; Alan Weiss, MD1; Larry Muenz, Ph.D2; Laurie Gould, BS*3
Published in the Journal of Chronic Fatigue Syndrome Vol. 8, No. 2, 2001. PP3-28.
[*Annapolis Research Center For Effective FMS/CFIDS Therapies, 466 Forelands Rd., Annapolis, MD 21401; 1) Anne Arundel Medical Center, Annapolis, MD; 2) Gaithersburg, MD; 3) USDA, Beltsville, MD]
No outside funding. Multivitamins supplied by Twinlab; Synthroid by Knoll; Fibrocare and Valerian Rest by To Your Health; Sporanox by Janssen; Oxytocin and DHEA by Belmar Pharmacy; Prozac by DISTA; Zoloft by Roerig; Paxil by SKB; Chromagen by Savage Labs; Serzone by Bristol-Myers Squibb; and Flagyl by Searle.
Abstract
Background
Hypothalamic dysfunction has been suggested in Fibromyalgia (FMS) and Chronic Fatigue Syndrome (CFS). This dysfunction may result in disordered sleep, subclinical hormonal deficiencies, and immunologic changes. Our previously published open trial showed that patients usually improve by using a protocol which treats all the above processes simultaneously. The current study examines this protocol using a randomized, double-blind design with an intent-to-treat analysis.
Methods
72 FMS patients (38 active: 34 placebo; 69 also met CFS criteria) received all active or all placebo therapies as a unified intervention. Patients were treated, as indicated by symptoms and/or lab testing, for: (1) subclinical thyroid, gonadal, and/or adrenal insufficiency, (2) disordered sleep, (3) suspected NMH, (4) opportunistic infections, and (5) suspected nutritional deficiencies.
Results
At the final visit, 16 active patients were "much better," 14 "better," 2 "same," 0 "worse," and 1 "much worse" versus 3, 9, 11, 6, and 4, respectively, in the placebo group (p < .0001, Cochran-Mantel-Haenszel trend test). Significant improvement in the FMS Impact Questionnaire (FIQ) scores (decreasing from 54.8 to 33.2 versus 51.4 to 47.7) and Analog scores (improving from 176.1 to 310.3 versus 177.1 to 211.9) (both with p < .0001 by random effects regression), and Tender Point Index (TPI) (31.7 to 15.5 versus 35.0 to 32.3, p < .0001 by baseline adjusted linear model) were seen. Long-term follow-up (mean 1.9 years) of the active group showed continuing and increasing improvement over time, despite patients being able to wean off most treatments.
Conclusions
Significantly greater benefits were seen in the active group than in the placebo group for all primary outcomes. Using an integrated treatment approach, effective treatment is now available for FMS/CFS.
Introduction
Fibromyalgia (FMS), which currently affects an estimated 3 to 6 million Americans,1,2 and Chronic Fatigue Syndrome (CFS) are two illnesses which often coexist. Severe persistent fatigue, diffuse migratory pain, cognitive dysfunction, and disordered sleep are common symptoms that patients often report in these overlapping syndromes. Current research suggests that many triggers can initiate a cascade of events, causing hypothalamic-target gland axis dysfunction3,4 and associated loss of normal circadian cycling of cortisol secretion.5 Hypothalamic dysfunction may result in some of the changes reported in FMS and/or CFS. These include:
- Disordered sleep6,7 with associated pain.8 Disordered sleep (as well as hormonal and other changes) may cause immune dysfunction-e.g., Natural Killer Cell dysfunction,9 decreased proliferative responses10 and opportunistic infections.6,11
- Hormonal deficiencies and hypothalamic-pituitary-target gland axis dysfunction.3,4,6,12 These can also contribute to the neurotransmitter changes seen in FMS.13 And,
- Autonomic dysfunction-including Neurally Mediated Hypotension (NMH).14,15
Macro and micro nutrient deficiencies have also been shown by some authors.16,19 In our initial pilot study,20 we explored the side effects, dosing and effectiveness of simultaneously treating the above problems. We found that simultaneously treating these resulted in significant clinical improvement. Which mix of treatments were needed, however, varied from patient to patient.
Although a concept that is sometimes uncomfortable and foreign to traditional styles of thinking, the need for multiple interventions can occur when an illness affects a critical control center (such as the hypothalamus) which impacts the multiple systems noted above. Unfortunately, we have not yet found a single treatment that reverses hypothalamic dysfunction directly. Thus, this situation is different from illnesses that affect a single target organ and which can be treated with a single intervention. For example, pituitary dysfunction itself often requires treatment with several hormones. This effect is multiplied in hypothalamic dysfunction, which affects several critical systems in addition to the pituitary gland. We therefore hypothesized that an integrated treatment approach based on simultaneously treating the above problems (even if a modest degree of suspicion that would usually not be treated is present) will be clinically beneficial in CFS and FMS. Subgroup analysis was done to assess the effect of antidepressant therapy. Our current study tests the efficacy of this therapeutic approach and the above hypothesis using a randomized, double-blind, placebo-controlled protocol with an intent-to-treat analysis in an outpatient setting.
Materials and Methods
Inclusion Criteria
Seventy-two patients with FMS who met entry criteria were entered into the study between November 1995 and November 1997. All but three (all in the active group) also met the 1994 Center For Disease Control (CDC) criteria for CFS.21 Patients were recruited by word of mouth, patient support groups, and media reports regarding our research center. All patients were required to meet 1990 American College of Rheumatology (ACR) criteria for FMS.22 Patients were not considered study candidates if major intercurrent illnesses (e.g., active cancer, multiple sclerosis, poorly controlled Diabetes, Emphysema, or Lupus) were present that could cause their symptoms. In addition, patients were excluded if: they were overtly hypothyroid (i.e., low T4 and elevated Thyroid Stimulating Hormone [TSH]) or hyperthyroid (i.e., high T4 and low TSH). Creatinine >1.9 mg/dL (168 umoL/L), AST >60 u/L (1.00 ukat/L), glucose > 200 mg/dL (11.1 mmol/L), Hematocrit (HCT) < .34 or Erythrocyte Sedimentation Rate (ESR) >45 mm/h were present. Patients were not excluded for depression, anxiety or sleep disorders.
Patients discontinued any previous treatments when able (except thyroid hormones, estrogen and progesterone) that were part of the study protocol. Patients were allowed to continue or begin active treatment upon completing the study and to participate in any other interventions on their own that were not part of the study protocol. Patients received a thorough history, physical exam and lab testing including a Complete Blood Count (CBC), Chem 18, serum magnesium, ESR, Urinalysis with micro, B12, Folate, Total T3, Free T4 or Free T7 index, TSH, HgbA1C, Cortrosyn (25 unit) Stimulation test, DHEA-Sulphate, IgE and stool O & P's. Follicle Stimulating Hormone (FSH), Luteinizing Hormone (LH) and estradiol levels were checked in females. Free Testosterone levels and stool tests for Clostridium difficile toxin were checked in a subset of the patients. Detailed informed consent was obtained from each patient.
Patient Population
Patient demographics at study entry are described in Table 1. Mean age at entry was 44.6 years (std. dev. 8.1, range 23-61). Sixty-six of 72 patients (92%) were female, and mean reported duration of CFS was 8.3 years (std. dev. 6.5, range 0.5-34 years). Average number of physicians consulted before coming to this clinic was 7.7 (range 0-100). Placebo patients were four years older, on average, than active-treatment patients (p = 0.037 by t-test), but there were no other significant demographic differences. The two treatment groups had no significant, or nearly significant, differences in mean entry values of the outcome measures, including the individual components of the Analog Total. With a possible range of 0-500, entry visit mean Analog Total was 176.5 (std. dev. 64.1, range 20-355) and, with a possible range of 0-80, the entry visit mean Fibromyalgia Impact Questionnaire score was 53.2 (std. dev. 9.6, range 30.4-74.6). Seventy-two patients met entry criteria and began treatment. Thirty-eight patients were randomized to the active intervention and 34 to the placebo intervention. The treatment protocol described below was completed by 32 patients in each group. The remaining 8 (6 active, 2 placebo) dropped out between visits 1 and 3. For some outcomes and visits, missing data yield sample sizes below 72 but, unless indicated, reported results concern the intention-to-treat sample. Participants gave written informed consent at the time of the initial examination and were informed of the double-blind, placebo-controlled nature of the study. The protocol is consistent with the principles of the Declaration of Helsinki.
Randomization and Blinding
Treatment was assigned in randomized blocks of six (B.B.). Patients then chose a date convenient for them to begin the study. Midway through the study, our statistician (L.M.), using the random number facility in SAS, generated the remaining code to maintain an equal number of active and placebo patients. Codes were kept away from the clinic in areas not accessible to patients or to the treating physician. Decisions as to whether the patients met entry criteria and their treatment prescriptions were made by the treating physician (J.E.T.), who was blinded to the patients' assignment and allocation sequence.
When possible, medications and identically appearing placebos were obtained from the companies making them. When not available, placebos were made by the pharmacist to approximate the medications' appearance. The treating physician did not have access to the medications. Containers of medications were labeled with various codes, with the code sheet accessible only to the pharmacist and the person responsible for dispensing medication (B.B.).
Outcome Measures
Four outcome measures were used. The primary outcome measures were the initial versus the final visit scores:
- Overall response—At the final visit the patients were asked whether they felt much worse, worse, same, better or much better after completing the protocol.
- Visual Analog (well-being) Scale (VAS) of 0-100 for 5 questions (obtained at each visit):
- How is your energy? 0 (near dead)-100 (excellent)
- How is your sleep? 0 (poor sleep)-100 (excellent, uninterrupted sleep)
- How is your mental clarity? 0 (severe "brain fog")-100 (normal healthy)
- How bad is your achiness? 0 (very severe, painful)-100 (no problem)
- How is your overall sense of well being? 0 (horrible)-100 (great)
- FIQ or Fibromyalgia Impact Questionnaire (disability index)-described previously23 (obtained at each visit).
- Tender Point Index (TPI)—This value is calculated by multiplying the number of positive tender points (TP—out of 18) by their degree of tenderness (1= TP painful, 2= grimaces, withdrawal or involuntary jerk on TP palpation, 3= markedly withdraws on palpation, 4= patient refuses to allow a TP to be examined because of the severity of the pain) (maximum score of 72). Five patients had their TPI checked 3 times (each 1 hour apart) at the initial visit, with TPI scores showing good intra-visit consistency. The TPI was assessed at the initial and final visits.
After the study was completed, overall response, Analog and FIQ scores were checked on all available patients (who opted to stay on treatment) to assess for tachyphylaxis and/or continuing improvement and the patient's ability to maintain their improvement after tapering off most of the treatments.
Treatment
It has been suggested that, in Myofascial Pain Syndrome (MPS), tissue needs for various hormones and nutrients are often greater than can be supplied by low-normal blood levels.24 Our initial study suggests that this also occurs in FMS.20 The symptom checklists we used,25 and a detailed discussion of our overall diagnostic and treatment protocols and the rationale behind them have been discussed and published previously.20,25,26 The protocol has also been integrated into a computerized algorithm.27 The specific (and less extensive) treatment protocol we used in this study is described in Table 2. Each patient received either all active or all placebo treatments as a unified intervention. How many active patients received each treatment and how decisions were made on which treatments to use in each individual patient is also described in Table 2.
Sample Size and Power
With a two-sided t-test, power 80% and type I error 5%, sample sizes of 38 and 34 allow detection of a standardized effect of 0.67, which is considered moderate. At the last visit, this effect corresponds to about 73 points and 11 points for the Analog Total and FIQ scales respectively-effects that we judged to be clinically significant.
Statistical Methods
Analog score totals and the FIQ, both of which were measured repeatedly, were compared between placebo and active treatment groups by two regression models, one for post-baseline trends in scores (random effects regression in SAS PROC MIXED, with time defined by visit number) and one for time to a 30% improvement over baseline scores, (i.e., a reduction for the FIQ and an increase for the Analog Total). Not all subjects had such changes, so the latter is a possibly-censored outcome and was analyzed by the Cox proportional hazards regression SAS PROC PHREG and by non-parametric Kaplan-Meier estimates implemented in SAS PROC LIFETEST, with time defined by elapsed days since study entry. Two random effects regression models were considered, one with treatment main effect only and one with both main effect and treatment by time interaction; the main effect estimates average post-baseline differences while the interaction assesses how rapidly treatment group means diverge. Stepdown likelihood ratio tests were used to select the best regression models. The Tender Point Index was recorded only at baseline and completion, and was analyzed by linear regression with baseline value as a covariate. The Cochran-Mantel-Haenszel test was used to compare treatments regarding the categorical Patient=s Summary. P < 0.05 was considered significant and statistical tests are two-sided, but without multiple comparison adjustments.
Timing of Visits and Duration of Follow-Up
Excluding a several-month time-period during which follow-up visits were unavailable, time trends in all analyses were based on elapsed days since study entry. Visits were scheduled one month apart and the median interval between consecutive visits was 31 days (26 and 37 days are the 25th and 75th percentile). The interval was nearly constant over the full period of follow-up. Subjects were followed for a median duration of 101 days and 96 days in active and placebo groups, respectively (25th percentiles: 88 and 89 days, 75th percentiles: 124 and 106 days).
Results
Analog Total and FIQ Scores
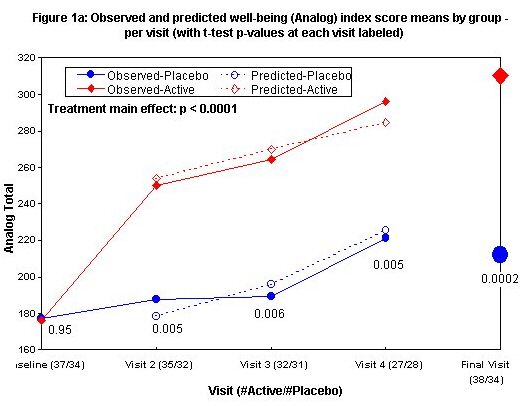
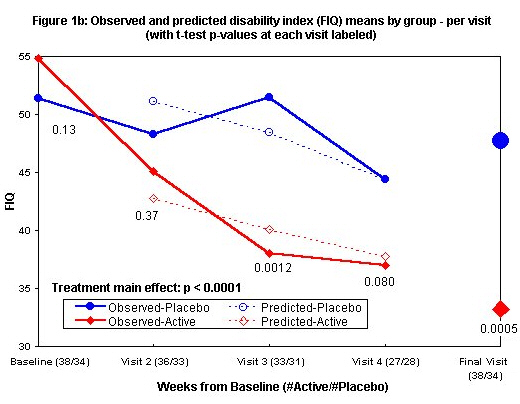
Means and standard deviations by visit are in Table 3. Figures 1a and 1b show both observed means and average predicted values from a random effect regression model, the latter only for visits 2 and later since baseline scores are predictors in the model. After adjustment for baseline score and age, the best model for Analog Total (Figure 1a) has only a significant main effect of the treatment (estimated effect 72, 95% Cl (37, 108), p < 0.0001 for a test of no difference). Mean Analog Total increases rapidly from visits 1 to 2 in the actively treated group and more slowly thereafter. After visit 2, there is a similar rate of improvement in the placebo group but not the early rapid increase seen in the actively treated group. The between-treatment difference of mean Analog Total scores was significant by visit 2 (two-sided p-value 0.005 by t test) and roughly constant thereafter. Also adjusting for baseline score and age, the best model for FIQ (Figure 1b) shows a significant main effect of treatment (estimated effect-11, 95% Cl [-16, -6], p < 0.0001 for a test of no difference). Mean FIQ declines slowly in the placebo group and more rapidly in the actively treated group with a significant difference seen by the third visit (two-sided p-value 0.0012 for t-test of no difference at visit 3). At the final visit, significant improvement in the FIQ (decreasing from 54.8 to 33.2 vs. 51.4 to 47.7) and Analog scores (improving from 176.1 to310.3 vs. 177.1 to 211.9) (p £ 0.0005 by unadjusted t-test comparing final scores and p < 0.0001 by random effects regression incorporating repeated measures for both FIQ and Analog) are seen.
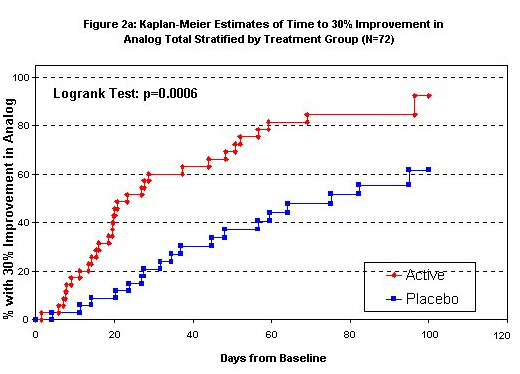
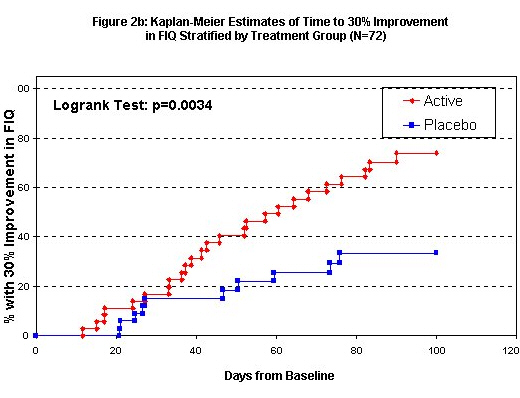
By treatment group, Kaplan-Meier curves of the time to 30% improvement in the two outcomes are seen in Figures 2a and 2b. Exact event times were interpolated between visits to identify when a 30% change was first seen. Events occurring after 100 days past baseline were truncated because of the small remaining sample size. For the Analog Total, 30/35 active group patients (86%, median time 22 days) improved by 30% while on study compared to 19/34 (56%, median time 70 days) of placebo group patients. Again, the time to 30% improvement is substantially and significantly shorter in the active group (log-rank test p-value 0.0006, Cox model p-value 0.0013 after adjustment for age and baseline Analog Total). For the FIQ 25/36 active group subjects achieved this improvement while on the study (69%, interpolated median time 58 days) compared to 11/34 placebo group subjects (34%, median time 101 days); the time to 30% improvement is substantially and significantly shorter in the active group (log-rank p-value 0.003, Cox model p-value 0.007 after adjustment for age and baseline FIQ).
Impact of Anti-Depressants on Study Outcomes
At some time during the study, Serotonin Uptake Inhibitors (SSRI's), Amitriptyline and Cyclobenzaprine were used by 76, 26, and 26% of the active group subjects and 74, 32, and 32% of the placebo group subjects. To address the possible impact of the non-randomized use of antidepressants on evidence of an overall treatment effect, random effects regressions tested the effect of the primary, randomized treatment adjusting for baseline score, visit, age, and the time-varying use of an SSRI, Amitriptyline, or Cyclobenzaprine. Antidepressant use was coded as three time-dependent covariates, each taking value 0 (no use or use stopped on that visit) or 1 (antidepressant prescribed or still in use) one visit before the Analog Total or FIQ outcome. In the placebo group, the antidepressants were shams so, for these subjects, a test of antidepressant effect compares a sham product to no product.
The regression models showed that SSRIs significantly decrease the FIQ (estimated 5.2 points improvement in sham or true SSRI users, p-value 0.029 for a test of zero effect) with no significant difference in the impact of the SSRI according to whether it was true or sham (p-value 0.55 for the interaction of treatment by SSRI). There were no other significant antidepressant effects in either treatment group on either Analog Total or FIQ. Furthermore, in models that adjusted for use of the three antidepressants, tests of the effect of the randomized treatment on the two primary outcomes remained highly significant (p < 0.0001 for both Analog Total and FIQ). Thus, these analyses identified a single significant effect of antidepressants with little impact on the primary comparisons of the active and placebo treatments.
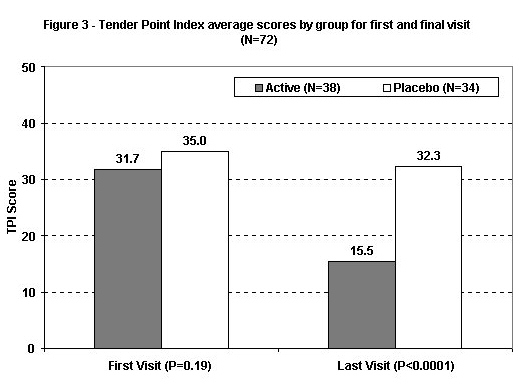
Tender Point Index and Patient Overall Response
At the last visit, the mean TPI (Figure 3) was significantly lower in the actively treated group (p < .0001 by t-test). A regression analysis showed that TPI score at the final visit was significantly related only to treatment and TPI score at entry and not to age or number of visits. At each TPI entry score, actively treated patients had mean adjusted scores 15.1 points lower than placebo patients (p < .0001).
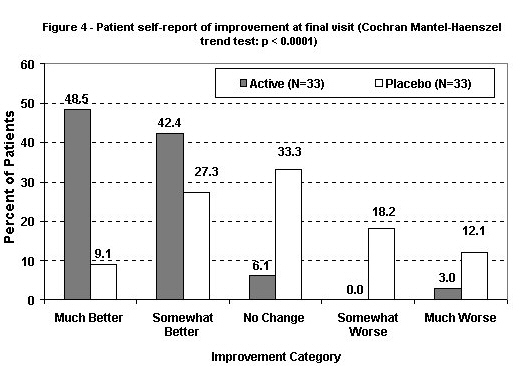
The distribution of patients overall response scores are in Figure 4 and Table 3 for 66 of 72 patients including all 64 patients who completed the study. Scores were significantly better among actively treated patients: p < .0001 by Cochran-Mantel-Haenszel trend test. If the ratings are assigned values from -2 (much worse) to +2 (much better), with zero for "same," mean scores were 1.33 (SD = 0.85) in the actively treated group and 0.03 (SD = 1.16) in the placebo group (p < .0001 by t-test). Overall response scores were missing for 6 patients, of which 5 were in the actively treated group (mean final Analog Total score of 214) and 1 was in the placebo group (final Analog Total score of 260). There is no evidence that the end-of-study summary was biased by the missing values.
Patients Meeting CFS Criteria and Patients Completing The Study
Conclusions for these patient subgroups (n = 69 and n = 64) were qualitatively the same as for the 72 patients in the intention-to-treat sample. For 69 patients in the intention-to-treat sample who met CFS criteria, random effects regression analyses for Analog Total and FIQ scores yielded estimated treatment effects of 71.5 and -11.4 points (p < .0001 for both), which are similar to findings for the 72 intention-to-treat patients. Results for the 64 patients that completed the study, including both effect sizes and p-values, were also similar to those for the 72 patients (details omitted).
Drop Outs
One patient in each group dropped out because of side effects and one in each group dropped out with no reason given. One active patient dropped out because "there were too many pills" and three active patients dropped out because they were "too busy" to be in the study (two of these because of new, severe illnesses in a family member).
Adverse events
By treatment group and body system, numbers of reported adverse events are given in Table 4. Patients were asked if they had complaints, but possible responses were not suggested. There were no significant differences between the treatment groups regarding any adverse event category although 7/38 active recipients compared to 2/34 placebo recipients reported a dermatological event (one-sided p = 0.10 by Fisher's exact test).
Pre and Post Study Cortrosyn Testing
Toward the end of the study 7 active patients given cortisol (and 13 given cortisol placebo) had post study Cortrosyn stimulation tests done. In the 7 active patients, average cortisol levels increased or stayed the same after treatment. Average cortisol levels (mcg/dL) pre, ½ hour and 1 hour post cortrosyn Intra-Muscularly (I.M.) were 14, 23, and 26 before treatment and 17, 23, and 26 after treatment. These results suggest that adrenal suppression did not occur with the low doses of cortisone used in the study.
Post Study Follow-Up
We were able to obtain follow-up data on 41 patients who chose to continue active treatment (many with their primary physicians) after the study. This data was obtained an average of 1.9 years after beginning active treatment. One had died (Melanoma). In the other 40, Analog and FIQ scores improved from 185 to 351 and 51.5 to 28.2 in those originally in the active group (180 to 308 and 51.4 to 36 in the total group). Of 38 patients for which overall response scores were available, 23 were "much better," 10 "better," 4 "same," 0 "worse," and 1 "much worse." The above includes 11 patients (10 from the placebo, 1 from the original active group) who were unable to get part of the treatment because of the medications' cost or because their primary care physician was unwilling to prescribe it.
Discussion
There are times that an illness occurs as a cascading series of events, where each dysfunction may trigger several others. We believe that this pathophysiology occurs in CFS/FMS. While these syndromes can be somewhat improved by treating a single underlying process, our pilot20 and current studies suggest that treatment is more effective when all of the processes are treated simultaneously as an integrated whole.
Immune dysfunctions have been suggested in CFS.9,10 In this current study, Clostridium difficile testing was positive in 11 out of 53 of the CFS/FMS patients we tested (20.7%), vs. a 2% prevalence in a healthy population.36 This may reflect both the host defense against opportunistic infections and the need for (often recurrent) antibiotics. Treating the various bowel infections frequently resolved severe gastrointestinal symptoms, often previously diagnosed as Irritable Bowel Syndrome, that had been present for years.
Non-restorative sleep is also suspected in FMS/CFS. Hypothalamic dysfunction can cause insomnia,6 which may be especially disruptive to slow-wave sleep. In healthy subjects, short-term sleep deprivation causes diminished cognitive function, decreased oral temperature and increased pain sensitivity. Experimental disruption of deep (slow-wave) sleep results in myalgias and fatigue.7 Clinical studies in FMS show that measures of pain and fatigue correlate with patients' assessment of sleep quality and improve with medications (e.g., amitriptyline and cyclobenzaprine) that restore stage 3 and stage 4 sleep.6 Sleep deprivation is immunosuppressive in animal models6,11 and may cause the decreased growth hormone levels seen in FMS patients.6
Unfortunately, most hypnotic sleep aids currently in use decrease deep stages of sleep. Zolpidem (Ambien), however, maintains deep (stage 3 and 4) sleep.37 Because zolpidem is short acting, and because of the severity of the disordered sleep, it may be necessary to add other sleep treatments (e.g., trazodone, clonazepam, and carisprodol—often in combination). These treatments are adjusted so that the patient gets 7-8 hours of uninterrupted sleep without waking or next day sedation.
Autonomic dysfunction (e.g., Neurally Mediated Hypotension or NMH) is also common in CFS,15,38 and may be ameliorated by increasing salt and water intake. Fludrocortisone (Florinef), may occasionally also improve NMH.
Some physicians may be uncomfortable with a study: 1) that uses multiple interventions adjusted for each patient and 2) that treats patients based on symptoms despite lab values being within the normal range. When possible, we prefer approaches without these difficulties. When not possible, it is important to remember that neither of these concerns has any significant impact on the scientific or clinical validity of the study data. It is, however, helpful to explore the rationale for using this approach in FMS/CFS.
Chronic unrelieved stress or distress (e.g., infectious, metabolic, situational, etc.) may "blunt" the stress response and its various axes and result in hypothalamic suppression. This may cause the cascade effect discussed in the introduction, and FMS/CFS may therefore effect multiple systems throughout the body. Each of these may then require simultaneous treatment. We believe that this mitigates the bias toward testing each individual treatment separately. It is helpful to remember that this bias comes from our having been trained in a period when a reductionistic approach was fashionable—and not because this approach holds any greater scientific validity.
Why then, would one treat for a process if the blood test is "normal?" Unlike primary organ failure, where the deficits eventually become marked, alterations in the patients' regulatory system can cause multiple marginal deficiencies, which, in the aggregate, may cause severe dysfunction. Much of our hormonal testing is based on primary gland failure and may not have been validated in conditions where blunting of the hypothalamic axes or peripheral resistance to hormone activity4,39,40 may occur. To use thyroid testing as one example, the TSH level, which is the only thyroid test that some physicians check in these illnesses, has been shown to have a blunted response to Thyrotropin Releasing Hormone (TRH) stimulation in FMS.4 Recent research suggests that normal thyroid lab tests are also often seen in the presence of multiple symptoms common in hypothyroidism,41 and that subclinical hypothyroidism is highly prevalent in some subgroups (e.g., elderly woman) with a concomitant increase in significant morbidity.42 In fact, in a Health Maintenance Organization HMO, when thyroid blood testing was ordered (i.e., even where it was likely that the ordering physician strongly suspected a thyroid disorder), only 3.2% (~2 Standard Deviation [SD]) of the tests showed overt hypothyroidism.43 The problem with using a lab standard of 2 SD, is that even though it's statistically useful, it may not be clinically appropriate. As Professor A.J. Padilla, of Einstein College of Medicine, notes, some disorders occur "in continuity" without a clear defining line between health and illness. "Physicians and normal persons tend to derive comfort from the ability to classify things based on objective criteria. In the case of disorders in continuity, this requires the establishment of arbitrary cutoffs to separate the well from the ill... This results in a trade off between sensitivity... and specificity." He notes four methodologies for defining this cutoff. Using 2 SD appears to be the least sensitive by far (one option is the top 10% [vs. 2 ½%] of the population)!44 This also suggests that our current lab norms, while possibly specific, may not be adequately sensitive and will miss many patients who might benefit from treatment. As has often been the case in medicine's history (e.g., diagnosing Angina based on symptoms before stress testing was available), physicians may need to rely on clinical information (e.g., weight gain, fatigue, myalgias, slow ankle reflex relaxation phase, etc., for hypothyroidism) to treat patients while waiting for the confirmatory technology to be developed and tested. Indeed, the importance of this concept is further supported by newer data that suggests that most patients who are clinically hypothyroid may have normal thyroid blood tests45 and, when treated with thyroxine, have significant clinical improvement!46 Indeed, when following thyroid therapy, thought-provoking work by Fraser, et al., suggests the possibility that "biochemical tests of thyroid function are of little, if any, value clinically" and that following clinical signs and symptoms may be more reliable.47
While recognizing our natural resistance to multiple treatments of perhaps subtle deficiencies, we also recognized the need to test, in a randomized, placebo-controlled trial, the clinical experience of many physicians who effectively treat these syndromes using the above approach. Despite these misgivings, the possible limitation of our tests' sensitivity, the relative safety of low dose hormonal supplementation,34,35 and the marked improvement in the severe debilitation experienced by many patients speak in favor of our moving beyond this resistance.
There are several other limitations to our study. Because inclusion criteria selected only adult FMS patients without other major intercurrent illnesses and only CFS patients who also had FMS, our results may not be generalizable to secondary FMS (e.g., patients who also have Lupus or Rheumatoid Arthritis), a pediatric population, or to CFS patients without FMS. We have found that these smaller subsets of patients usually improve with our treatment protocol, but have a higher incidence of treatment failures. We do not recommend that this protocol be used in patients with multiple sclerosis.
A second limitation was our inability to get exactly identical placebos for some study medications. Also, in any placebo-controlled study using psychoactive drugs, unblinding may occur because of the side effects of these medications. That there was no significant difference in the number of patients experiencing side effects between the two groups, however, suggests that unblinding did not occur. The treating physician did not have access to the medications during the study. In addition, at the final visit only 5 patients thought that they could tell if they were on active or placebo based on the medications' appearance or side effects. Of these, two guessed correctly and three incorrectly suggesting that blinding was effective.
A third concern is whether the benefit seen in our study was predominantly caused by SSRI and/or tricyclic use. Several other controlled studies have shown benefit with using tricyclics (e.g., amitriptyline or cyclobenzaprine).48 Unfortunately the benefit is often modest and may wane after 6 months.31 A report by Goldenberg, et al. showed that Fluoxetine and Amitriptyline (individually or combined) for 6 weeks, were much more effective than placebo32 Other controlled studies using SSRI's alone, however, did not show them to be of significant benefit relative to placebo.49,50,51 In our study, analysis of the data suggests that SSRI's and/or tricyclics were not significantly more effective than placebo and p values remained £ .0001 when our data was adjusted to exclude the effect of these agents.
A fourth limitation is the lack of objective measures to monitor the effectiveness of treatment. The impact of FMS/CFS may, however, be reasonably estimated by subjective symptoms. Thus, the patient's symptom assessment can be used as a reliable method to measure the treatments' effectiveness. Though no consensus yet exists on what the best outcome measures are for use in FMS/CFS studies, the FIQ has been validated23 and the VAS, TPI and patient overall responses are also commonly used.52
A fifth concern relates to whether the treatments' effectiveness might diminish over time. Although not blinded, our follow-up of patients an average of 1.9 years after beginning treatment showed that effectiveness of the active group's treatment increased over time and that this benefit persisted even after some or most of the treatment was (as per our protocol) terminated.
We hope this study will be helpful to physicians, patients and researchers studying FMS/CFS. Over time, treatment hopefully will be improved, markedly simplified and better understood. An independent, randomized, multi-center, replicative study of our findings is currently being developed. In the interim, this treatment protocol offers effective treatment for patients suffering with FMS/CFS.
Acknowledgements
We would like to thank Amy Podd of A.P. Business Solutions, Mary Groom and David He for their superb technical support.
We would like to dedicate this study to the memory of Janet Travell, MD, whose pioneering work in the treatment of Myofascial Pain Syndrome forms the foundation of our treatment protocol.
References
(1) Goldenberg DL. Fibromyalgia Syndrome-An Emerging but Controversial Condition. JAMA 1987; 257: 2782-2787.
(2) Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The Prevalence & Characteristics of Fibromyalgia in the General Population. Arthritis & Rheumatism 1995 Jan; 38 (1): 19-28.
(3) Demitrack MA, Dale JK, Straus SE, Laue L, Listwak SJ, Kruesi MJP, et al. Evidence for Impaired Activation of the Hypothalamic-Pituitary-Adrenal Axis in Patients with Chronic Fatigue Syndrome. J Clin Endocrinol Metab 1991; 73: 1224-1234.
(4) Neeck G, Riedel W. Thyroid Function in Patients With Fibromyalgia Syndrome. J Rheumatol 1992; 19: 1120-1122.
(5) McCain GA, Tilbe KS. Diurnal Hormone Variation in Fibromyalgia Syndrome: A Comparison With Rheumatoid Arthritis. J Rheumatol 1989; 16 Suppl 19: 154-157.
(6) Pillemer S, Bradley LA, Crofford LJ, Moldofsky H, Chrousos GP. The Neuroscience and Endocrinology of FMS-[An NIH] Conference Summary. Arthritis & Rheumatism Nov 1997; 40 (11): 1928-1939.
(7) Drewes AM, Nielson KD, Taagholt SJ, Bjerregaard K, Svendson L, Gade J. Slow Wave Sleep in FMS [Abstract]. J. Musculoskeletal Pain1995; 3 Suppl 1: 29.
(8) Older SA, Battafarano DF, Danning CL, Ward JA, Grady EP, Derman S. Delta Wave Sleep Interruption and FMS in Healthy Patients [Abstract]. J. Musculoskeletal Pain 1995; 3 Suppl 1: 159.
(9) Barker E, Fujimura SF, Fadem MB, Landay AL, Levy JA. Immunologic Abnormalities Associated with Chronic Fatigue Syndrome. Clin Infect Dis 1994; 18 Suppl 1: S136-S141.
(10) Straus SE, Fritz S, Dale JK, Gould B, Strober W. Lymphocyte Phenotype & Function in the Chronic Fatigue Syndrome. J Clin Immunol 1993; 13 (1): 30-40.
(11) Everson CA. Sustained Sleep Deprivation Impairs Host Defense. Am J Physiol 1993; 265 (Regulatory Integrative Comp. Physiol 34); R1148-R1154.
(12) Griep EN, Boersma JW, de Kloet ER. Altered Reactivity of the Hypothalamic-Pituitary-Adrenal Axis in the Primary Fibromyalgia Syndrome. J Rheumatol 1993; 20: 469-474.
(13) Hammond CB. Menopause and Hormone Replacement Therapy: An Overview. Obstet Gynecol 1996; 87 Supplement: 2S-15S.
(14) Rowe PC, Bou-Holaigah I, Kan JS, Calkins H. Is NMH an Unrecognized Cause of Chronic Fatigue? Lancet 1995; 345: 623-624.
(15) Bou-Holaigah I, Rowe PC, Kan J, Calkins H. The Relationship Between NMH and the CFS. JAMA 1995; 274: 961-967.
(16) Cox IM, Campbell MJ, Dowson D. Red Blood Cell Magnesium & Chronic Fatigue Syndrome. Lancet 1991; 337: 757-760.
(17) Romano TJ, Stiller JW. Magnesium Deficiency in Fibromyalgia Syndrome. J Nutr Med 1994; 4: 165-167.
(18) Evengard B, Nilsson CG, Astrom G, Lindh G, Lindqvist L, Olin R, et al. Cerebral Spinal Fluid Vitamin B12 Deficiency in Chronic Fatigue Syndrome [Abstract]; Proceedings of The American Association for Chronic Fatigue Syndrome Research Conference; 1996 Oct 13-14; San Francisco, California.
(19) Regland B, et al. Increased Concentrations of Homocysteine in the Cerebrospinal Fluid in Patients With Fibromyalgia and Chronic Fatigue Syndrome. Scandinavian Journal of Rheumatology 1997, 26: 301.
(20) Teitelbaum J, Bird B. Effective Treatment of Severe Chronic Fatigue: A Report of a Series of 64 Patients. J Musculoskeletal Pain 1995; 3 (4): 91-110.
(21) Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A, et al. The Chronic Fatigue Syndrome: A Comprehensive Approach to its Definition & Study. Ann Intern Med 1994; 121: 953-959.
(22) Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia-Report of the Multicenter Criteria Committee; Arthritis & Rheumatism 1990 Feb; 33 (2): 160-172.
(23) Burckhardt CS, Clark SR, Bennett RM. The Fibromyalgia Impact Questionnaire: Development and Validation. J of Rheumatology 1991; 18: 728-734.
(24) Travell JG, Simons DG. The Trigger Point Manual, Volume 1. Baltimore (MD): Williams & Wilkins; 1983.
(25) Teitelbaum J. From Fatigued To Fantastic!. 2nd ed. Garden City Park (NY); Avery Press; 1996.
(26) Teitelbaum J. From Fatigued To Fantastic Newsletter. Annapolis (MD); Deva Press; 1997-1999.
(27) www.endfatigue.com.
(28) Zhdanova IV, Wurtman RJ, Lynch HJ, et al. Sleep Inducing Effects of Low Doses of Melatonin Ingested in the Evening. Clin Pharmacol Ther 1995; 57: 552-558.
(29) Dressing H, et al. Insomnia: Are Valerian/Melissa Combinations of Equal Value to Benzodiazepine? Therapiewoche 1992; 42: 726-736.
(30) Bennett RM, Gatter RA, Campbell SM, Andrews RP, Clark SR, Scarola JA. A Comparison of Cyclobenzaprine & Placebo in the Management of Fibrositis-A Double-Blind Controlled Study. A & R Primary Care Review 1990 July-Aug; 2 Suppl 4: 16-24.
(31) Carette S, Bell MJ, Reynolds WJ, et al. Comparison of Amitriptyline, Cyclobenzaprine & Placebo in the Treatment of Fibromyalgia-A Randomized, Double-Blind Clinical Trial. Arthritis and Rheumatism 1994: 37: 32-40.
(32) Goldenberg D, Mayskiy M, Mossey R, Ruthazer R, Schmid C. A Randomized, Double-Blind Crossover Trial of Fluoxetine and Amitriptyline in the Treatment of FMS. Arthritis and Rheumatism 1996; 39 Suppl 11: 1852-1859.
(33) Lindenbaum J, Rosenberg IH, Wilson PWF, Stabler SP, Allen RH. Prevalence of Cobalmin Deficiency in the Elderly Framingham Population. Am J Clin Nutr 1994; 60: 2-11.
(34) Jefferies WM. Low-dosage Glucocorticoid Therapy. Arch Intern Med 1967; 119: 265-278.
(35) Jefferies WM. Safe Uses of Cortisol. 2nd ed. Springfield (Illinois): Charles C. Thomas; 1996.
(36) Wainstein MA, Resnick MI. Managing Nosocomial Infection With C. Difficile. IM Nov 1995: 15-22.
(37) Merlotti L, Roehrs T, Koshorek G, Zorick F, Lamphere J, Roth T. The Dose Effects of Zolpidem on the Sleep of Healthy Normals. J Clin Psychopharmacol 1989; 9 (1): 9-14.
(38) Freeman R, Komaroff AL. Does the Chronic Fatigue Syndrome Involve the Autonomic Nervous System? The American Journal of Medicine 1997 April; 102: 357-363.
(39) Lowe JC, Garrison RL, Reichman AJ, Yellin J, Thompson M, Kaufman D. Effectiveness and Safety of T3 Therapy for Euthyroid Fibromyalgia: A Double-Blind, Placebo-Controlled Response Driven Crossover Study. Clinical Bulletin of Myofascial Therapy 1997: 2 (2/3): 31-58
(40) Lowe JC, Reichman AJ, Yellin J. The Process of Change During T3 Treatment for Euthyroid Fibromyalgia: A Double-Blind, Placebo-Controlled, Crossover Study. Clinical Bulletin of Myofascial Therapy 1997; 2 (2/3): 91-124
(41) Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado Thyroid Disease Prevalence Study. Archives of Internal Medicine; 2000 Feb 28; 160: 526-534.
(42) Hak AE, Pols HAP, Visser TJ, Drexhage HA, Hofman A, Witteman JCM. Subclinical Hypothyroidism is a Risk Factor For Atherosclerosis & M.I. in Elderly Women. Ann Intern Med 2000; 132: 270-278.
(43) Nordyke RA, Reppun TS, Madanay LD, Woods JC, Goldstein AP, Miyamoto LA. Alternative Sequences of Thyrotropin and Free Thyroxine Assays for Routine Thyroid Function Testing. Arch Intern Med 1998; 158: 266-272.
(44) Padilla AJ. Who Has Diabetes? Cortlandt Forum, 2000 Feb; p110-111.
(45) Skinner GRB, Thomas R, Taylor M, Bolt S, Krett S, Wright A, et al. Thyroxine Should be Tried in Clinically Hypothyroid but Biochemically Euthyroid Patients. BMJ 14 June 1997; Volume 314.
(46) Skinner GRB, Holmes D, Ahmad A, Davies JA, Benitez J. Clinical Response to Thyroxine Sodium in Clinically Hypothyroid Biochemically Euthyroid Patients. J Nutritional And Environmental Medicine 2000; 20: 115-124.
(47) Fraser WD, Biggart EM, O'Reilly DJO, Gray HW, McKillop JH, Thomson JA. Are Biochemical Tests of Thyroid Function of Any Value in Monitoring Patients Receiving Thyroxine Replacement? Br Med J 1986 Sept; 293: 808-810.
(48) Goldenberg D, Fibromyalgia Syndrome a Decade Later. Arch Intern Med 1999; 159: 777-785.
(49) Wolfe F, Cathey MA, Hawley DJ. A Double-Blind Placebo Controlled Trial of Fluoxetine in Fibromyalgia. Scand J Rheumatol 1994: 23: 255-259.
(50) Norregaard J, Volkmann H, Danneskiold-Samsoe B. A Randomized Controlled Trial of Citalopram in the Treatment of Fibromylagia. Pain 1995: 61: 445-449.
(51) Vercoulen JH, Swanink CM, Zitman FG, Vreden SG, Hoofs MP, Fennis JF, et al. Randomized, Double-Blind, Placebo-controlled Study of Fluoxetine in Chronic Fatigue Syndrome. Lancet 1996 Mar 30; 347(9005): 858-861.
(52) Goldenberg D. Treatment of Fibromyalgia Syndrome. Rheumatic Disease Clinics of North America 1989 Feb; 15(1): 61-71.
Table 1. Patient Demographics
|
|
||
| Variable | Active N=38 |
Placebo N=34 |
|
|
||
| Age in Years-Average (Range) Standard Deviation |
42.7 (28-58) 6.5 |
46.7 (23-61) 9.2 |
|
|
||
| Sex Percentage-Female Male |
92% 8% |
91% 9% |
|
|
||
| Length of Fatigue in Years-Average (Range) Standard Deviation |
7.1 (.5-18) 4.8 |
9.7 (1-34) 7.8 |
|
|
||
| Onset-Percentage-Gradual Sudden |
42 58 |
35 65 |
|
|
||
| # of Doctors Seen Previously For Symptoms Average (Range) Standard Deviation |
6.3 (0-20) 4.8 |
9.2 (1-100) 16.7 |
|
|
||
Table 2. Treatment Protocol
Patients received all active or all placebo treatments as a single intervention.
Table 2a: Medicines That All Patients Received
| For Sleep: | ||
|
|
||
| A | Melatonin 3/10 mg P.O. QHS28 and | |
| B | Valerian 180 mg/Melissa 90 mg combination (Valerian Rest by To Your Health), 1-2 tablets P.O. QHS29 | |
|
Plus the below treatments as needed to result in 7-8 hours of solid sleep without waking or next-day sedation. Mixing of a low dose of several medications was used instead of a high dose of a single agent in order to decrease next-day sedation. |
||
| A | Zolpidem (Ambien) 10 mg, ½-1 ½ P.O. QHS and/or | |
| B | Trazodone (Desyrel) 25-200 mg P.O. QHS and/or | |
| C | Cyclobenzaprine (Flexeril) 10 mg, ½-2 P.O. QHS30,31 and/or | |
| D | Carisprodol (Soma) 350 mg, ½-1 P.O. QHS and/or | |
| E | Amitriptyline (Elavil) 10 mg, ½-5 P.O. QHS31,32 and/or | |
| F | Clonazepam (Klonopin) ½ mg, ½-8 tablets P.O. QHS | |
|
|
||
| For nutritional support (these two supplements are used long-term): | ||
|
|
||
| A | Daily One Cap Multivitamin (Twinlab), 1 tablet P.O. QAM | |
| B | Magnesium with malic acid (Fibrocare by To Your Health), 2 tablets p.o. Tid | |
|
|
||
Table 2b: Treatments That Were Individualized Based on Test Results or Clinical History
| Treatment: | If: | ||||||
|
|
|||||||
| Ferrous Fumarate (Chromagen) 1 P.O. QD between 2 and 6 PM on an empty stomach. | Ferritin £ 40 ng/mL (ug/L) or iron % saturation £ 22%. | ||||||
|
|
|||||||
| B12 1,000 mcg/cc, 1cc I.M. 1-3x a week for 12 doses then PRN or B12 1,000 mcg SL QD (if patient was unable to obtain injections). | B12 level < 540 pg/mL (398 pmoL/L).18,19,33 | ||||||
|
|
|||||||
| Levothyroxine (Synthroid) 25 mcg, 1-4 QAM or dessicated thyroid (Armour) 30 mg ½-3 tablets QAM (adjust to a clinically optimal dose based on relief of symptoms while keeping the free T4 within normal range). | If TSH > 2.5 or < .9 U/mL and/or total T3 is < 95 ng/dL (1.5 nmoL/L) and/or free T4 is < 1.0 ng/dL (13 pmoL/L) and patient has 3 of the following symptoms: weight gain, oral temp < 98.3°, dry skin, thin hair, constipation, achiness, and/or cold intolerance. | ||||||
|
|
|||||||
| Cortisol (Cortef) 5 mg, 1-3 tabs QAM, ½-1 ½ tabs at noon and ½ tab at 4 PM, using lowest clinically optimal dose (usual dose 5-12 ½ mg/day—up to 20-25 mg/d).34,35 | Cortrosyn stimulation test with cortisol baseline £ 12 ug/dL, (33 1nmoL/L) and/or ½ hour increases < 7 ug/dL (193 nmoL/L), or 1 hour increase < 11 ug/dL (303 nmoL/L) with a 1 hour cortisol level < 28 ug/dL (773 nmoL/L) or HgbA1C < 5.1% and/or patient has 3 of the following: sugar craving, shakiness relieved by eating, dizziness, moodiness, recurrent infections that persist longer than expected, high stress at illness onset or low B/P. | ||||||
|
|
|||||||
| DHEA 5-50 mg P.O QD (decrease the dose if acne or darkening of facial hair in females) occurs. | DHEA-Sulphate (mcg/dl) (x.02714=umoL/L) | ||||||
|
|
|||||||
| In Males: | In Females: | ||||||
| DHEA-Sulphate | RX (mg/d) | DHEA-Sulphate | RX (mg/d) | ||||
| umoL/L | mcg/DL | umoL/L | mcg/DL | ||||
| 0-2.7 | 0-100 | 50 | 0-0.8 | 0-30 | 25 | ||
| 2.8-5.4 | 101-200 | 40 | 0.9-2.2 | 31-80 | 20 | ||
| 5.5-7.6 | 201-280 | 25 | 2.3-3.0 | 81-110 | 10 | ||
| 7.7-8.7 | 281-320 | 10 | 3.1-3.8 | 111-114 | 5 | ||
|
|
|||||||
| Testosterone Enanthate (Delatestryl) 100 mg I.M. QWK (in males) or natural Testosterone 2 mg P.O. QD or BID in females. | Free testosterone in lowest quintile for age. | ||||||
|
|
|||||||
| Estrogen replacement (in females) offered to patient:26 if < 40 Y.O.-Ovcon 35, if > 40 Y.O. or side effects on Ovcon, Estradiol 1/2-2 mg QD or Triestrogen (10% Estradiol, 10% Estrone, 80% Estriol) 1¼-5 mg/d P.O. on day 1-25 of cycle and (if uterus present) natural progesterone 100 mg P.O. qhs or 200 mg P.O. qhs day 16-25 of cycle. | Estradiol < 75pg/mL (275pmoL/L) and/or FSH & LH > 10 mI.U./mL (I.U./L) and/or irregular periods, hot flashes, inadequate vaginal lubrication, low libido, flaring of FMS symptoms before periods or S/P TAH or tubal ligation. | ||||||
|
|
|||||||
| Oxytocin 10 units P.O. QD | Severe cold hands /feet and pallor. | ||||||
|
|
|||||||
| Fludrocortisone (Florinef) .1 mg/d (and increase dietary salt, water & potassium) beginning at ¼ tab/day & increasing by 1/4 a tab Q 3-7 days | B/P < 100/60, or orthostatic dizziness or FMS symptoms worsened by standing against wall for 10 minutes. | ||||||
|
|
|||||||
| Sertraline (Zoloft) 50 mg, 1/2-2 QHS OR Paroxetine (Paxil) 20 mg, 1/2-2 QAM OR Fluoxetine (Prozac) 20 mg, 1-2 QAM OR Nefazodone (Serzone) 100 mg B.I.D. | If NMH symptoms above, depression or persistent severe pain. | ||||||
|
|
|||||||
| Nystatin 500,000 units 2 P.O. T.I.D. x 3-5 months plus, in more severe cases, Itraconazole (Sporanox) 100 mg 2 P.O. QD with food x 6-12 weeks (begin 4 weeks after Nystatin begun). Do not take Seldane, Hismanal, Propulsid or antacids with Itraconazole. | If stool microscopic exam showed higher than normal fungal levels or symptoms suggesting fungal overgrowth (e.g., thrush, recurrent yeast vaginitis or antibiotic use, onchomycosis)—by questionnaire.25 | ||||||
|
|
|||||||
| Metronidazole (Flagyl) 250 mg P.O. QID x 10 days. or 750 mg P.O. TID x 10 days followed by iodoquinol (Yodoxin) 650 mg P.O. TID. | If stool was positive for Clostridium difficile. or If other Metronidazole (Flagyl) sensitive parasites were present. | ||||||
|
|
|||||||
| Doxycycline 100 mg P.O. B.I.D. x 6 weeks. | Recurrent body temperatures >98.6 °F. | ||||||
|
|
|||||||
Table 2C. Number of Patients on Each Treatment (at Some Time During the Study) Out of 38 Active Patients
| Treatment | # of Patients on Treatment |
|
|
|
| Daily One Multivitamin | 38 |
|
|
|
| Valerian Rest | 38 |
|
|
|
| Magnesium/Malic Acid (Fibrocare) | 38 |
|
|
|
| Melatonin 3/10 mg | 38 |
|
|
|
| Chromagen (iron) | 24 |
|
|
|
| Vitamin B-12 | 30 |
|
|
|
| SSRI (Sertraline, Paroxetine, Fluoxetine, Nefazodone) | 29 |
|
|
|
| Amitriptyline (Elavil) | 10 |
|
|
|
| Cyclobenzaprine (Flexeril) | 10 |
|
|
|
| Desyrel | 24 |
|
|
|
| Ambien | 23 |
|
|
|
| Klonopin | 8 |
|
|
|
| Soma | 22 |
|
|
|
| Synthroid | 18 |
|
|
|
| Armour Thyroid | 15 |
|
|
|
| Cortef | 29 |
|
|
|
| DHEA | 24 |
|
|
|
| Florinef | 19 |
|
|
|
| Oxytocin | 15 |
|
|
|
| Estrace | 7 |
|
|
|
| Triestrogen | 6 |
|
|
|
| Progesterone | 9 |
|
|
|
| Testosterone | 12 |
|
|
|
| Nystatin | 35 |
|
|
|
| Sporanox | 27 |
|
|
|
| Flagyl | 10 |
|
|
|
| Doxycycline | 4 |
|
|
|
Table 3. Summary of FMS Treatment Outcomes Among 72 Patients
| Analog Scales, Totals, By Visit | ||||||||
|
|
||||||||
| Time | Active | Placebo | P-Value | |||||
|
|
||||||||
| N | Mean | Std. Dev | N | Mean | Std. dev | |||
|
|
||||||||
| 1 | 37 | 176.1 | 70.3 | 34 | 177.1 | 57.6 | .95 (1) | |
|
|
||||||||
| 2 | 35 | 249.7 | 88.0 | 32 | 187.5 | 87.3 | ||
|
|
||||||||
| 3 | 32 | 264.1 | 115.2 | 31 | 189.2 | 93.4 | ||
|
|
||||||||
| 4 | 27 | 295.7 | 90.0 | 28 | 221.3 | 99.5 | <.0001 (2) | |
|
|
||||||||
| Last | 38 | 310.3 | 111.3 | 34 | 211.9 | 103.7 | .0002 (1) | <.0001 (3) |
|
|
||||||||
| FIQ Scale, By Visit | ||||||||
|
|
||||||||
| 1 | 38 | 54.8 | 10.3 | 34 | 51.4 | 8.4 | .14 (1) | |
|
|
||||||||
| 2 | 36 | 45.1 | 15.1 | 33 | 48.3 | 14.1 | ||
|
|
||||||||
| 3 | 33 | 38.0 | 17.7 | 31 | 51.5 | 14.0 | ||
|
|
||||||||
| 4 | 27 | 37.0 | 15.5 | 28 | 44.4 | 15.3 | <.0001 (2) | |
|
|
||||||||
| Last | 38 | 33.2 | 18.2 | 34 | 47.7 | 15.5 | .0005 (1) | <.0001 (3) |
|
|
||||||||
| TPI | ||||||||
|
|
||||||||
| First | 38 | 31.7 | 10.5 | 34 | 35.0 | 10.6 | .19 (1) | |
|
|
||||||||
| Last | 32 | 15.5 | 9.5 | 30 | 32.3 | 11.4 | <.0001 (1) | <0.0001 (3) |
|
|
||||||||
| Patient's Overall Response | ||||||
|
|
||||||
| Much Better | Better | Same | Worse | Much Worse | p-value | |
|
|
||||||
| Active | 16 | 14 | 2 | 0 | 1 | <.0001 (4) |
|
|
||||||
| Placebo | 3 | 9 | 11 | 6 | 4 | |
|
|
||||||
|
||||||
|
|
||||||
Table 4. Side Effects
| Side Effect Categories | Active N=38 (Number of patients with side effects) |
Placebo N=34 (Number of patients with side effects) |
|
|
||
| Dermatological | 7 | 2 |
|
|
||
| Psychological | 12 | 8 |
|
|
||
| Gastrointestinal | 9 | 11 |
|
|
||
| Autonomic Dysfunction | 6 | 3 |
|
|
||
| Sleep Changes | 3 | 3 |
|
|
||
| Miscellaneous | 9 | 5 |
|
|
||
| Total number of patients in group to report any side effect. | 24 | 22 |
|
|
||
Graphs